What is Stage III Melanoma?
Stage III melanomas are tumors that have spread to regional lymph nodes or have developed in-transit deposits of disease, but there is no evidence of distant metastasis. Stage III melanoma is regional melanoma, meaning it has spread beyond the primary tumor (local) to the closest lymph nodes, but not to distant sites. There are four subgroups of Stage III melanoma: IIIA, IIIB, IIIC, IIID. Stage III is invasive melanoma.
- Subgroups are IIIA, IIIB, IIIC, IIID
- Stage III melanoma is defined by four primary characteristics
- Important distinction within Stage III: whether the spread to lymph nodes can be detected microscopically or macroscopically
- Microscopically, also called clinically occult = seen by pathologist during biopsy or dissection;
- Macroscopically, also called clinically detected = seen by naked eye or felt by hand or seen on CT scans or ultrasound
- Risk: Intermediate to high for regional or distant spread
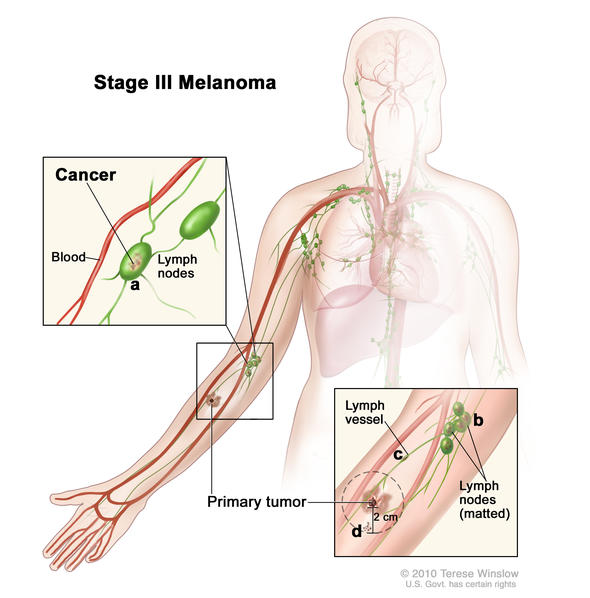
Characteristics of Stage III Melanoma
Stage III melanoma is defined by four characteristics:
- Primary tumor depth and ulceration
- Number of lymph nodes to which it has spread
- Whether the tumor spread to the lymph node is clinically occult or clinically apparent
- Clinically occult tumors are so tiny they are not visible to the naked eye. They can be detected only by microscopic evaluation after sentinel lymph node biopsy or elective lymph node dissection
- Clinically detected (or apparent) tumors can be felt during physical examination or seen with the naked eye when inspected by a surgeon or pathologist. They can also be detected by imaging like CT scans and ultrasound. Their presence is confirmed by lymph node dissection or when the tumor is seen to extend beyond the lymph node capsule.
- Presence of tumor deposits outside of the primary tumor including in-transit, satellite, or microsatellite metastases
Subgroups of Stage III Melanoma
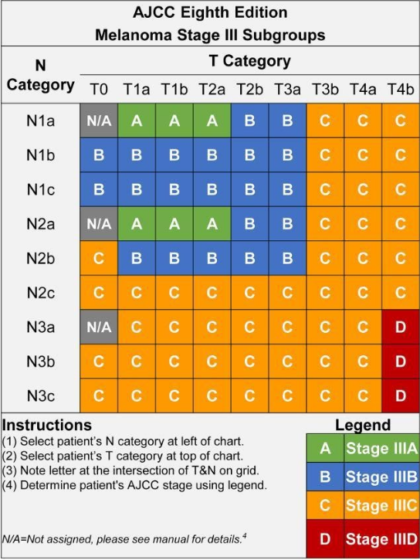
There are four subgroups of Stage III melanoma (IIIA, IIIB, IIIC, and IIID). The subgroups are defined by the TNM categories, so in order to understand the subgroups, it’s helpful to understand the TNM categories. This next section is quite detailed, but you’ll see there’s a clear pattern.
TNM Categories and Subcategories for Stage III Melanoma
T means Tumor. This category is related to your primary melanoma tumor.
T0 means no evidence of a primary tumor.
The T1 category includes tumors that are less than 1.0 mm thick. T1 subcategories:
- T1a tumors are less than 0.8 mm thick and are not ulcerated.
- T1b tumors are less than 0.8 mm thick and are ulcerated; or are 0.8 to 1.0 mm thick and can be ulcerated or not.
The T2 category includes tumors that are greater than 1.0 mm and up to 2.0 mm thick. T2 subcategories:
- T2a tumors are greater than 1.0 mm and up to 2.0 mm thick and do not have ulceration.
- T2b tumors are greater than 1.0 mm and up to 2.0 mm thick and are ulcerated.
The T3 category includes tumors that are 2.0 to 4.0 mm thick. T3 subcategories:
- T3a tumors are 2.0 to 4.0 mm thick and are not ulcerated.
- T3b tumors are 2.0 to 4.0 mm thick and are ulcerated.
The T4 category includes tumors that are greater than 4.0 mm thick. T4 subcategories:
- T4a tumors are greater than 4.0 mm thick and are not ulcerated.
- T4b tumors are greater than 4.0 mm thick and are ulcerated.
N means Node. This category is related to the regional spread of your melanoma, beyond the primary tumor.
The N1 category comprises spread to only one lymph node; OR there is in-transit, satellite, or microsatellite metastasis. N1 subcategories:
- N1a means that the one positive lymph node was clinically occult.
- N1b means that the one positive lymph node was clinically detected.
- N1c means no lymph nodes were positive, but there is in-transit, satellite, or microsatellite metastasis.
The N2 category comprises spread to two or three lymph nodes; OR that there is in-transit, satellite, or microsatellite metastases AND one positive lymph node. N2 subcategories:
- N2a means that the two to three positive lymph nodes were clinically occult.
- N2b means that the two to three lymph nodes were clinically detected.
- N2c means one lymph node was positive, either clinically occult or clinically detected AND there is in-transit, satellite, or microsatellite metastasis.
The N3 category comprises spread to four or more lymph nodes OR there is in-transit, satellite, and/or microsatellite metastases with two or more positive lymph nodes OR any number of matted nodes without or with in-transit, satellite, and/or microsatellite metastasis. N3 subcategories:
- N3a means that the four or more positive lymph nodes were clinically occult.
- N3b means that the four or more positive lymph nodes were clinically detected.
- N3c means two or more lymph nodes were positive, either clinically occult or clinically detected AND/OR there are any number of matted nodes AND there is in-transit, satellite, and/or microsatellite metastasis.
M means distant Metastasis. Stage III melanoma does not, by definition, have distant metastasis, so a Stage III patient will be M0—no evidence of distant metastasis.
Look at your most recent pathology report and find your TNM score. (For a primer on how to understand your pathology report, read here.) Identify your T subcategory and your N subcategory scores. Then look at the chart, below, to find your Stage subgroup. For example, if your TNM score is T2aN2a, that means your tumor was greater than 1.0 mm and up to 2.0 mm thick and was not ulcerated, and you have two to three positive lymph nodes that were clinically occult. You’ll see on the chart that you would be deemed Stage IIIA.
Risk: Even after surgical treatment, Stage III disease has an intermediate to high risk for local recurrence or distant metastasis. Within Stage III, the earlier the melanoma is found and treated, the better the outcome.
Treatments for Stage III Melanoma
Stage III melanoma has multiple treatment options and can include surgery (including sentinel lymph node biopsy and possibly completion lymph node dissection), neo-adjuvant therapy, adjuvant therapy, radiation therapy, and clinical trials. You will likely see a surgical oncologist for the surgery-related treatments and a medical oncologist for the drug-related treatments. If you have any radiation treatments, you will see a radiation oncologist.
It is important to know whether all of your Stage III melanoma has been completely removed with surgery (known as “resected Stage III”), or if it was not possible to remove all of the melanoma (known as “unresectable Stage III”). These two types of Stage III melanoma are treated very differently. Unresectable Stage III patients are treated similarly to Stage IV melanoma patients. Read about Stage IV melanoma.
Order of Treatment
Patients with melanoma often receive more than one type of treatment, and certain terms are used to describe the order of treatments given. Neo-adjuvant treatment is what is given before primary treatment—in melanoma, primary treatment is generally surgery—to shrink tumors. For Stage III patients, neo-adjuvant treatment is mostly given in clinical trials. Primary treatment is the main treatment to remove cancer. Adjuvant treatment is given after primary treatment to kill any remaining cancer cells. FDA-approved adjuvant therapies for Stage III are noted below.
Surgery
The standard treatment for all primary melanoma is a surgery called wide local excision. The purpose of the surgery is to remove any cancer remaining after the biopsy of the primary tumor.
In a wide local excision, the surgeon removes any remaining tumor from the biopsy site, the surgical margin (a surrounding area of normal-appearing skin), and the underlying subcutaneous tissue, to make certain the whole tumor has been removed.
The width of the margin taken depends upon the thickness of the primary tumor. The surgical margin guidelines adopted and recommended by the National Comprehensive Cancer Network (NCCN) for wide local excision of the primary melanoma range from 0.5 cm to 2 cm:
Tumor Thickness
Surgical Margin
In Situ
0.5-1.0 cm
Less than or equal to 1 mm
1.0 cm
Greater than 1.0mm to 2.0 mm
1.0-2.0 cm
Greater than 2.0 to 4.0 mm
2.0 cm
Greater than 4.0 mm
2.0 cm
Sentinel Lymph Node Biopsy (SLNB)
Sentinel lymph node biopsy is generally performed to determine whether the closest lymph nodes to the primary tumor are cancerous—in other words, to determine if your tumor is Stage III. If you have already been diagnosed with Stage III melanoma, a SLNB is typically recommended only when it is suspected there might be melanoma in another lymph node basin.
The results of the biopsy will guide the course of treatment.
Lymph Node Dissection
When cancerous lymph nodes are found via SLNB, an additional surgery called completion lymph node dissection (CLND) to remove the remaining lymph nodes from the area may be recommended. In the past, CLND was performed somewhat routinely after a positive SLNB, but in a recent study of more than 1900 patients, it was determined that completion lymph node dissection (CLND) does not prolong survival. Your doctor may discuss CLND with you if your SLNB found one or more positive nodes.
Surgery to remove all area lymph nodes is also considered when lymph nodes are noted on exam or several enlarged lymph nodes are noted on imaging. In this case, the surgery is called therapeutic lymph node dissection (TLND). It is performed to possibly stop the spread of the disease to distant sites.
Adjuvant Therapy
Adjuvant means aiding or assisting, and adjuvant therapy is the term for treatment given after the primary treatment—surgery—to remove melanoma. Systemic treatment is often recommended as the adjuvant treatment for Stage III melanoma. These systemic therapies are administered either by pill or infusion and enter into the bloodstream in an effort to reach and destroy any remaining cancer cells in the body.
The following are adjuvant therapy options approved by the FDA for Stage III patients with resected melanoma, meaning the melanoma was completely removed with surgery:
SINGLE AGENT IMMUNOTHERAPIES
Purpose: Keytruda is a humanized monoclonal antibody. It is designed to block a cellular target known as PD-1, which restricts the body’s immune system from attacking melanoma cells.
How it works: Keytruda is a humanized monoclonal antibody that works by increasing the ability of the body’s immune system to fight advanced melanoma. Keytruda blocks the interaction between PD-1 and its lignds, PD-L1 and PD-L2, and may affect both tumor cells and healthy cells.
Which patients: Keytruda is approved for patients with Stage IIB and IIC melanoma following a complete resection, Stage III melanoma, and Stage IV melanoma.
How it is given: Keytruda is given intravenously, each 2mg/kg dose is given over a 30-minute period, usually every 3 weeks until there is disease progression or unacceptable side effects. It is given in an outpatient clinic and does not require hospitalization.
Effectiveness: In a large Phase 1 clinical trial, half of the participants received Keytruda at the recommended dose of 2mg/kg. Of these patients, approximately 24 percent had their tumors shrink. This effect lasted at least 1.4 to 8.5 months and continued beyond this period in most patients. A similar percentage of patients had their tumor shrink at the 10mg/kg dose.
An improvement in survival or disease-related symptoms has not yet been established. Merck is currently conducting ongoing Phase 2 and 3 clinical studies in advanced melanoma patients.
Side effects: Although it is not common, Keytruda can cause your immune system to attack normal organs and tissues in many areas of your body and can affect the way they work. These problems can sometimes become serious or life-threatening.
Important: Call or see your doctor right away if you develop any symptoms of the following problems or these symptoms get worse:
Lung problems (pneumonitis). Symptoms of pneumonitis may include:
- shortness of breath
- chest pain
- new or worse cough
Intestinal problems (colitis) that can lead to tears or holes in your intestine. Signs and symptoms of colitis may include:
- diarrhea or more bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems (hepatitis). Signs and symptoms of hepatitis may include:
- yellowing of your skin or the whites of your eyes
- dark urine
- nausea or vomiting
- feeling less hungry than usual
- pain on the right side of your stomach area (abdomen)
- bleeding or bruising more easily than normal
Hormone gland problems (especially the thyroid, pituitary, and adrenal glands). Signs and symptoms that your hormone glands are not working properly may include:
- rapid heart beat
- weight loss
- increated sweating
- weight gain
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- muscle aches
- dizziness or fainting
- headaches that will not go away or unusual headache
Kidney problems, including nephritis and kidney failure. Signs of kidney problems may include:
- change in the amount or color of your urine.
Problems in other organs.
- rash
- changes in eyesight severe or persistent muscle or joint pains
- severe muscle weakness
The following are the common side effects (>_20):
- fatigue
- cough
- nausea
- severe Itching
- rash
- decreased appetite
- constipation
- joint pain
- diarrhea
Patient assistance: Patients can call 1-855-257-3932 or visit www.merckaccessprogram-keytruda.com
Purpose: Opdivo is a humanized monoclonal antibody. It is designed to block a cellular target known as PD-1, which results in an anti-tumor immune response.
How it works: Opdivo is a humanized monoclonal antibody that works by increasing the ability of the body’s immune system to fight advanced melanoma. Opdivo blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response.
Which patients: Opdivo is approved for Stage III patients with lymph node involvement who have undergone a complete resection or Stage IV melanoma patients.
How it is given: Stage III patients are given Opdivo intravenously, with each 240mg dose given over a 30-minute period every 2 weeks or 480 dose given over a 60-minute period every 4 weeks, until disease recurrence or unacceptable side effects for up to 1 year. It is given in an outpatient clinic and does not require hospitalization.
Stage IV patients are given Opdivo intravenously, with each 240mg dose given over a 30-minute period every 2 weeks or 480 dose given over a 60-minute period every 4 weeks, until disease progression or unacceptable side effects. It is given in an outpatient clinic and does not require hospitalization.
Effectiveness: Stage III patients: In a phase III clinical trial, patients who were treated with Opdivo had a 35% reduction in the risk of recurrence or death versus those who received Yervoy.
Stage IV patients: In a phase III clinical trial, patients who were treated with Opdivo had a 45% reduction in the risk of disease progression versus those who received Yervoy. At the 5-year follow-up, the overall survival rate was 44%.
Side effects: Although it is not common, Opdivo can cause your immune system to attack normal organs and tissues in many areas of your body and can affect the way they work. These problems can sometimes become serious or life-threatening.
Important: Call or see your healthcare provider right away if you develop any symptoms of the following problems or these symptoms get worse:
Lung problems (pneumonitis). Symptoms of pneumonitis may include:
- New or worsening cough
- Chest pain
- Shortness of breath
Intestinal problems (colitis) that can lead to tears or holes in your intestine. Signs and symptoms of colitis may include:
- Diarrhea (loose stools) or more bowel movements than usual
- Blood in your stools or dark, tarry, sticky stools
- Severe stomach area (abdomen) pain or tenderness
Liver problems (hepatitis). Signs and symptoms of hepatitis may include:
- Yellowing of your skin or the whites of your eyes
- Severe nausea or vomiting
- Pain on the right side of your stomach-area (abdomen)
- Drowsiness
- Dark urine (tea colored)
- Bleeding or bruise more easily than normal
- Feeling less hungry than usual
Kidney problems, including nephritis and kidney failure. Signs of kidney problems may include:
- Decrease in the amount of urine
- Blood in your urine
- Swelling in your ankles
- Loss of appetite
Hormone gland problems (especially the thyroid, pituitary, and glands). Signs and symptoms that your hormone glands are not working properly may include:
- Headache that will not go away or unusual headaches
- Extreme tiredness
- Weight gain or weight loss
- Changes in mood or behavior, such as decreased sex drive, irritability or forgetfulness
- Dizziness or fainting
- Hair loss
- Feeling cold
- Constipation
- Voice gets deeper
Problems in other organs. Signs of these problems include:
- Rash
- Changes in eyesight
- Severe or persistent muscle or joint pains
- Severe muscle weakness
- The most common adverse reaction (reported in at least 20% of patients) was rash.
Patient assistance: 1-800-861-0048 (hours: 8 am – 8 pm est.) or www.bmsaccesssupport.com
Purpose: Yervoy is an anti-CTLA-4 monoclonal antibody. It is designed to restore and strengthen the immune system by supporting the activation and proliferation of T-cells, a critical component of the immune system. By supporting T-cells, Yervoy helps sustain an active immune response to fight the cancer cells. In Stage III patients, it helps lower the risk that the melanoma will return following surgery.
How it works: Yervoy is a human monoclonal antibody designed to block the activity of a molecule called CTLA-4, a protein that normally helps keep the immune system cells, called T cells, in check. When Yervoy blocks CTLA-4, the drug “takes the breaks off the immune system” and allows T cells to activate and proliferate in order to attack the melanoma cells.
Which patients: Yervoy is approved for patients with Stage III or Stage IV melanoma.
How it is given: Yervoy is given intravenously. It is given in an outpatient clinic, and does not require hospitalization. For adjuvant melanoma: 10 mg/kg is administered over 90 minutes every 3 weeks for 4 doses, followed by 10 mg/kg every 12 weeks for up to 3 years or until documented disease recurrence or unacceptable toxicity. For unresectable or metastaic melanoma: 3 mg/kg is administered intravenously over 90 minutes every 3 weeks for a total of 4 doses.
Effectiveness: In a large clinical trial, Stage IV patients who were treated with Yervoy plus GP100, a peptide vaccine, showed a significant improvement in overall survival versus those who received GP100 alone. Higher estimated survival rates were observed at one year (46% vs. 25%) and at two years (24% vs.14%). Yervoy improved overall median survival by four months.
In a 2013 analysis of data collected from 12 prospective and retrospective studies of 1861 patients, it was shown that the median overall survival for patients treated with Yervoy was 11.4 months. Among these patients, 22% were still alive after three years. There were no deaths among patients who survived beyond seven years, at which time the overall survival rate was 17%.
In a large clinical trial, patients with Stage III melanoma received either Yervoy or a placebo after complete surgical removal of their melanoma. At a median 5.3 years follow-up, the 5 year overall survival for patients receiving Yervoy was 65.4% compared to 54.4% for the patients receiving the placebo. Treatment with Yervoy reduced the risk of death by 28%.
Side effects: Because Yervoy makes T cells more responsive to many stimuli (not just cancer cells), the drug can cause powerful autoimmune reactions in which the immune system attacks normal cells in the body. 15% of patients reported autoimmune reactions that were classified as severe and some fatalities did occur in the studies of Yervoy.
Colitis (inflammation of the colon), occurs in about 12% of patients. In 5% of the patients the symptoms are moderate, while in 7% of the patients the symptoms can be severe or life threatening, causing death in less than 1% of the cases. Signs and symptoms of colitis are:
-
- diarrhea (loose stools) or more bowel movements than usual
- blood in your stools or dark, tarry, sticky stools
- stomach pain (abdominal pain) or tenderness
Important: If you develop diarrhea, you should call your doctor immediately. If you cannot reach your doctor go to the nearest emergency room. For the majority of patients, if found early, diarrhea can be controlled within a few days.
Hepatitis: An inflammation of the liver that occurs in less than 5%-10% of cases, but can lead to liver failure. Since it rarely has symptoms, it is important that your liver function be tested before you start Yervoy and during treatment, to identify any elevation in liver enzymes. Signs and symptoms of hepatitis may include:
- yellowing of your skin or the whites of your eyes
- dark urine (tea colored)
- nausea or vomiting
- pain on the right side of your stomach
- bleeding or bruising more easily than normal
Important: Before each dose of Yervoy, your blood should be checked for liver function.
Skin/Toxicity/Rash: Occurs in about 50% of patients. Typically, it is a rash that comes and goes without itch. However, it can present as a more severe skin reaction (toxic epidermolysis necrolysis). Signs and symptoms of a severe skin reaction are:
- skin rash with or without itching
- sores in your mouth
- skin blisters and/or peels
Inflammation of Hormone Glands: Dysfunction of the pituitary, adrenal, or thyroid glands, which occurs less than 10% of the time. Signs and symptoms that your glands are not working properly include:
- persistent or unusual headaches
- unusual sluggishness, feeling cold all the time, or weight gain
- changes in mood or behavior such as decreased sex drive, irritability, or forgetfulness
dizziness or fainting
Important: The most common way inflammation of the hormone glands is determined is through blood work. Your physician should monitor this at regular intervals. If you have a preexisting thyroid problem before you start this medicine, it’s even more important to watch your blood counts and to make your physician aware of this so that this can be safely monitored.
Other Side Effects:
Inflammation of the Nerves: This can lead to paralysis. Symptoms of nerve problems may include:
- unusual weakness of legs, arms, or face
- numbness or tingling in hands or feet
Inflammation of the Eyes: Symptoms may include:
- blurry vision, double vision, or other vision problems
- eye pain or redness
Most Common Side Effects:
The most common side effects of YERVOY include: tiredness, diarrhea, itching, rash, headache, weight loss and nausea.
Important: Women who are pregnant should not take Yervoy because it may cause harm to a developing fetus.
Financial Support: Click here
Y.E.S. Program: Click here
Risk Evaluation and Mitigation Strategy (REMS Program): Click here
Combination Therapies
Purpose: Mekinist in combination with Tafinlar blocks a cellular pathway to inhibit the growth of melanoma metastases.
How it works: Tafinlar is an inhibitor of some mutated forms of BRAF kinases. Mekinist is an inhibitor of some MEK kinases. Both of these kinases are key proteins that help melanoma cells grow. About half of all patients with melanoma have a mutated form of the BRAF protein in their tumors.
Which patients: Mekinist in combination with Tafinlar is indicated for the treatment of Stage III patients with lymph node involvement who have undergone a complete resection and Stage IV patients, with BRAF V600 E or V600 K mutations. Before starting treatment the patient should test positive for the presence of a BRAF V600E or V600K mutation.
How it is given: Tafinlar and Mekinist are both oral drugs.
Stage III: Patients receive 150mg of Taflinar orally twice daily and receive 2mg of Mekinist once daily until disease recurrence or unacceptable side effects for up to 1 year.
Stage IV: Patients receive 150mg of Taflinar orally twice daily and receive 2mg of Mekinist once daily until disease recurrence or unacceptable side effects.
Effectiveness: Stage III: In a Phase III trial that compared the Mekinist and Taflinar combination to a placebo the 3-year relapse-free survival rate in the combination arm was 58% vs 39% in the placebo arm. This represented a 53% reduced risk of disease recurrence or death.
Stage IV: In a Phase III trial that compared the Mekinist and Tafinlar combination to Zelboraf, at 1year the overall survival in the combination arm was 73% versus 64% in the Zelboraf arm. After 2 years, 51% of patients taking Tafliinar plus Mekinist were alive compared to 38% taking Zelboraf alone.
Progression-free survival (the time a patient lives without cancer growing or spreading) was 12.6 months for patients treated with the Mekinist and Tafinlar combination, versus 7.3 months for patients treated with Zelboraf.
Side effects: Several side effects are common in the course of treatment. Most of these side effects are mild or moderate, although there have been some severe side effects. These side effects can usually be managed with either a decrease in the dosage or by briefly stopping one or both of the drugs and then resuming after the symptoms improve. Sometimes, when the drug or drugs are resumed, it is at a lower dosage.
New Skin Cancers
- For patients treated with Mekinist in combination with Tafinlar, the incidence of Cutaneous Squamous Cell Carcinoma was 7% compared with 19% in patients treated with Tafinlar alone. The incidence of basal cell carcinomas was higher (9% compared with 2%) for Mekinist in combination with Tafinlar.
New Primary Melanomas: they occur in approximately 2% of patients.
- If these lesions appear, they should be removed, but typically you can continue taking the drug without changing the dosage.
Important: You should have a dermatologic examination before you start these medicines, and every two-three months while you remain on the drugs, up to 6 months after you stop taking the drugs.
Serious Fevers: 26% of all patients developed fevers when treated with Tafinlar alone. In 2% of these patients these fevers were serious and were accompanied by one or more additional symptoms. For patients treated with Mekinist in combination with Tafinlar, the incidence of fever was 71%, while 25% had other associated symptoms such as:
- Low Blood Pressure
- Chills
- Dizziness
- Too much fluid loss (dehydration)
- Kidney problems
Important: Tell your doctor immediately if you get a fever.
Skin rash is the most common side effect, occurring in 65% of all patients taking Mekinist in combination with Tafinlar. In some cases it can be severe enough to cause hospital admission. Tell your doctor if you get any of these symptoms:
- Skin rash
- Acne
- Blistering
- Redness, swelling, peeling, or tenderness of hands or feet
- Skin redness
Hemorrhage: 16% of all patients had a hemorrhagic event (including accumulation of blood within the brain) and in 4% of cases it was fatal.
Blood Clots: 7% of all patients had a blood clot in a vein (deep vein thrombosis) or in the lung (pulmonary embolism). One patient died of a pulmonary embolism (2% of patients).
Important: Immediately seek medical attention if you experience any concerning symptoms, including but not limited to; shortness of breath, chest pain, leg swelling, bleeding or confusion.
Heart problems, including heart failure, occurred in approximately 9% of patients. Signs and symptoms of heart problems include:
- Feeling like your heart is pounding
- Shortness of breath
- Swelling of ankles or feet
- Feeling lightheaded
Important: You should have your heart function checked before you start taking Tafinlar in combination with Mekinist as well as during treatment.
Blood Sugar Problems (diabetes): Some people may develop high blood sugar, or if they have diabetes it may get worse. Tell your doctor if you have any of the following symptoms:
- Increased thirst
- Urinating more than usual
- Your breath smells like fruit
Eye problems such as uveitis, retinal vein occlusion, and Retinal Pigment Epithelial Detachment occurred very rarely in patients. Symptoms include eye pain, swelling or redness, blurred vision, floaters, or sudden loss of vision.
Important: You should have your eyes examined before and while you are taking these medicines. Tell your doctor immediately if any eye symptoms appear.
Patient assistance: Patients can call 1-800-282-7630
Yervoy (ipilimumab), Keytruda (pembrolizumab), Opdivo (nivolumab) are immunotherapies—treatments designed to boost the immune system to fight the return of melanoma.
Targeted therapies and targeted therapy combinations work by blocking the function of the mutated BRAF protein. Targeted therapies are only available to those who have the BRAF mutation in their tumors.
There are multiple Stage III treatment options. AIM at Melanoma has written a guide to help you understand the options available and how to weigh those options with your oncology team. We recommend all Stage III patients read this guide.
As noted above, patients with unresectable Stage III melanoma, meaning that it was not possible to remove all of the melanoma, are treated similarly to Stage IV melanoma patients. To learn what treatment options are available for Stage III unresectable melanoma read here.
Clinical Trials
Clinical trials are research studies to evaluate new therapies and improve cancer care. These studies are responsible for most of the advances in cancer prevention, diagnosis, and treatment. You may be eligible to participate in a clinical trial. Several treatments for Stage IV melanoma are currently being tested in clinical trials for Stage III. Sometimes the trial is evaluating a new drug or a new combination of drugs; sometimes the trial is evaluating a different dosage or dose schedule for an existing drug. Other trials are evaluating neo-adjuvant treatments for Stage III melanoma.
Radiation Therapy
For those with multiple or large cancerous nodes, radiotherapy can be used after surgery to prevent the tumors from returning at that site, but radiation does not impact survival or the spread of tumors to other sites.
What to Ask Your Doctor About Stage III Melanoma
When your doctor tells you that you have Stage III melanoma, it can be frightening and overwhelming. But it is important to use the time with all of your doctors to learn as much about your cancer as you can. Your doctors will provide you important information about your diagnosis, prognosis, and treatment options.
It is often helpful to bring a friend or family member with you to your doctor appointments. This person can lend moral support, ask questions, and take notes.
The following questions are those you may want to ask your doctors. Some of the questions are for your medical oncologist, some are for your surgical oncologist, and some for your dermatologist. Remember, it is ALWAYS okay to ask your doctor to repeat or clarify something s/he has said so that you can better understand it. You may find it helpful to print out these questions and bring them with you to your next appointment.
