Immune Checkpoint Inhibition for Melanoma: A Focus on Veterans
Introduction
Veterans have been shown to have a higher risk for melanoma compared to the general U.S. population due to sun exposure and lack of protection against ultraviolet (UV) radiation during active military service. Results from a recent study with 54,554 nonveterans and 6,753 veterans showed that there was a higher prevalence of any skin cancer history among US veterans compared with nonveterans (9.0% vs 2.9%) and a higher prevalence of melanoma history (2.2% vs 0.6%).1
Immunotherapy has been shown to be very effective in the treatment of melanoma. This article will provide a general overview of a type of immunotherapy known as immune checkpoint inhibitors (ICIs). In addition, this article will review what we currently know about ICIs for the treatment of melanoma in the veteran population.
Understanding Immunotherapy
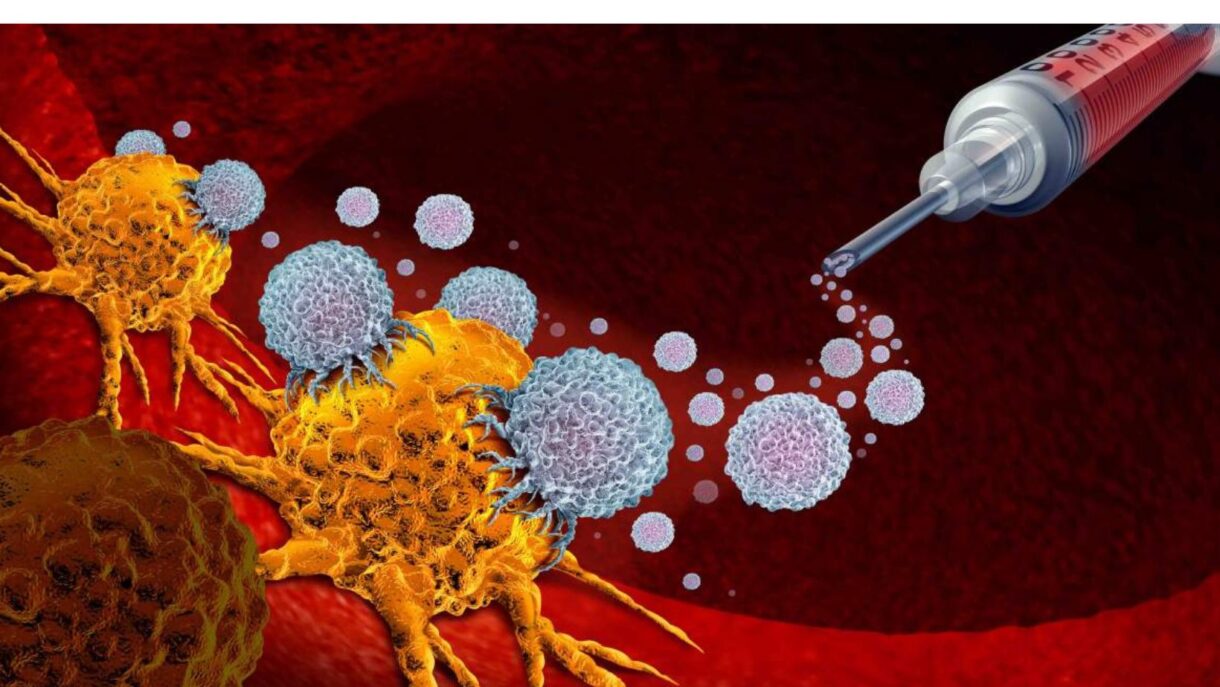
Many people with healthy immune systems still develop cancer. Sometimes the immune system doesn’t see the cancer cells as foreign because the cells aren’t different enough from normal cells, and sometimes the immune system recognizes the cancer cells, but the response might not be strong enough to destroy the cancer. Immunotherapy is a treatment approach that uses parts of your immune system to fight diseases such as melanoma by stimulating your own immune system (T cells, B cells, and natural killer cells) to work harder or smarter to attack cancer cells.
“Veterans face higher melanoma risk due to sun exposure and lack of UV protection during military service.”
Immune Checkpoints and Checkpoint Inhibitor Therapies2
Our immune system has “checkpoints” that regulate our immune response and ensure that healthy cells are not destroyed. In general, they work like this: Immune cells express immune checkpoint protein receptors that can recognize their counterparts (called ligands) on normal and tumor cells. If these receptors find their ligands and interact in a way similar to a lock and key mechanism, the checkpoint activates and starts shutting the immune response off. If these receptors do not find their ligands, the checkpoint is not activated, and the immune system launches an immune response.
These mechanisms are important for us to stay healthy because they allow the body to keep immune cells active when needed and to shut them off if they are activated inappropriately. Unfortunately, cancer cells exploit these mechanisms. Cancer cells often express the ligands of immune checkpoints, and when the receptors interact with them, the immune cells turn off instead of attacking the growing tumor.
When immune cells encounter melanoma cells, they may express a protein receptor called PD-1. In addition, melanoma tumor cells may express the ligand of PD-1—called PD-L1. If the PD-1 receptor interacts with the PD-L1 ligand, the immune checkpoint PD-1 is tricked into stopping the immune response and the cancer grows in an uncontrolled way. A protein called CTLA-4 may also be expressed when immune cells encounter melanoma cells, which also halts the immune response.
Immunotherapies known as immune checkpoint inhibitors (ICIs) are designed to halt the activation of immune checkpoints and reinvigorate immune cells to fight cancer. Examples are ipilimumab (Yervoy), which blocks CTLA-4, and nivolumab (Opdivo) and pembrolizumab (Keytruda), which both block PD-1 on immune cells binding to PD-L1 on cancer cells.
Checkpoint inhibitor therapies have been a major advance for patients with melanoma. Sometimes they are used as monotherapy (for example, nivolumab alone or pembrolizumab alone) and other times they are used in combination (for example, ipilimumab and nivolumab together). Recent long-term data show that the melanoma-specific survival rate in the CheckMate 067 clinical trial at 10 years was 52% with nivolumab-plus-ipilimumab, 44% with nivolumab, and 23% with ipilimumab.3 In addition, the melanoma-specific survival rate in the KEYNOTE-006 trial at 10 years was 45.2% for pembrolizumab and 31.3% for ipilimumab.4
“Immune checkpoint inhibitors reinvigorate immune cells to attack cancer, counteracting the ways melanoma cells evade immune detection.”
Immune Checkpoint Inhibitors in Veterans
Veterans Affairs patients have been historically underrepresented in national datasets and clinical trials.5,6 As a result, there is a need for real-world studies outside of clinical trials to provide information on treatment outcomes in this patient population. Results from two of these studies, and what we can conclude from them, are described here.
Retrospective Electronic Health Record Data Study7
Outcomes across multiple tumor types at the VA were investigated in a retrospective study using electronic health record data from VA facilities nationwide. The VA population has patients who are frequently under-represented in clinical trials, including those who are older, frailer, and more racially heterogeneous than patients included in typical clinical trials. In this study, a total of 11,888 patients who received ICIs were identified. This included 1,706 patients (14.4%) with melanoma.
One question that is partially answered by the results of this study is how veterans with melanoma and other cancers fare on ICIs and how this compares to results from clinical trials. It’s important to know that survival rates in clinical trials can be higher than those seen outside of clinical trials, primarily because only certain patients are selected for trials, and often those with higher disease burden, comorbidities, etc., are excluded. The veteran population tends to be older, sicker, and more diverse than the general population, so it’s important to understand outcomes specifically for veterans.
Patients specifically with melanoma who received first-line nivolumab had the longest survival across cancers in the study; median survival was 25.5 months after ICI initiation. This result is important because it supports the effectiveness of ICI monotherapy in the veteran patient population with higher risk characteristics than those seen in clinical trials. Typically, ICI monotherapy has been shown to be less effective compared to combination therapy in clinical trials, and it is important to note that ICI combination therapy can be associated with more serious and more frequent side effects,3 which is a significant concern in patients such as veterans with higher risk characteristics.
Real-World Effectiveness Study8
Results from another study help us understand treatment response in a high-risk population of veterans with melanoma who received ICI monotherapy and ICI combination therapy. A research letter was published in the Journal of the American Academy of Dermatology about effectiveness of ICIs among veteran patients with melanoma in the adjuvant setting (when ICIs are given after surgery to remove melanoma) and in the neoadjuvant systemic therapy setting (when ICIs are the first treatment given before surgery). In total, 677 patients with melanoma from the Veterans Health Administration were included. In this study, the median age was 68 years old, most patients were male (98%), and the majority of patients were White (96%). Patients also had many comorbidities, such as congestive heart failure, diabetes, and hypertension. Anti-PD-1 monotherapy was the most commonly used first-line treatment in both the adjuvant (66.7%) and neoadjuvant systemic therapy settings (50.3%).
In the adjuvant setting, for melanoma-specific survival (which takes into account death only from melanoma), 91.2% of patients treated with anti-PD-1 monotherapy were survivors five years after diagnosis and therapy initiation compared to 53.2% of patients who received anti-CTLA-4 monotherapy. These results are similar to those from studies with non-veterans that show higher survival with anti-PD1 therapy compared with anti-CTLA-4 therapy.3 No patients in this study received combined immune checkpoint inhibitors in the adjuvant setting.
In the neoadjuvant systemic therapy setting, for melanoma-specific survival, 70.4% of patients who received anti-PD-1 monotherapy survived three years after diagnosis and therapy initiation compared to 47.9% of patients who received anti-CTLA-4 monotherapy and 64.7% in patients who received anti-PD-1/anti-CTLA-4 combination therapy.
Survival was higher with anti-PD1 monotherapy compared to anti-CTLA-4 monotherapy in the adjuvant setting. In addition, survival was higher with anti-PD1 monotherapy and anti-PD1/anti-CTLA-4 combination therapy compared to anti-CTLA-4 monotherapy in the neoadjuvant setting. Similar to the results from the other study described here, the effectiveness of anti-PD-1 monotherapy in veterans was supported.
Conclusions
Immunotherapy with ICIs has been shown to be effective in patients with melanoma in clinical trials. Results described here in with veteran patients similarly show that ICIs are effective in this patient population. Results from the studies show that ICI monotherapy is effective in the veteran population that includes patients who are older, frailer, and more racially heterogeneous than patients included in typical clinical trials. These results support that ICI monotherapy is an effective option for higher risk patients who may not be able to tolerate combination therapy, which may cause more side effects and more serious side effects compared to monotherapy. It will be beneficial to learn more about treatment outcomes with ICIs in veterans and to determine if there are any other differences compared to patients from clinical trials and from the general population. It will also be important to focus on strategies to improve ICI treatment response in veterans in the future.
REFERENCES
1. Rezaei SJ, Kim J, Onyeka S, et al. Skin Cancer and Other Dermatologic Conditions Among US Veterans. JAMA Dermatol. 2024;160(10):1107-1111.
2. He X, Xu C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020;30(8):660-669.
3. Wolchok JD, Chiarion-Sileni V, Rutkowski P, et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2025;392(1):11-22.
4. Long GV, Carlino MS, McNeil C, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. 2024;35(12):1191-1199.
5. Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results Registry. J Am Acad Dermatol. 2022;87(1):72-79.
6. Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The impact of underreported Veterans Affairs data on National Cancer Statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101(7):533-536.
7. La J, Cheng D, Brophy MT, et al. Real-World Outcomes for Patients Treated With Immune Checkpoint Inhibitors in the Veterans Affairs System. JCO Clin Cancer Inform. 2020;4:918-928.
8. Kim DY, Swetter SM, Huhmann L, et al. Real-world effectiveness of immune checkpoint inhibitors and BRAF/MEK inhibitors among veteran patients with cutaneous melanoma. J Am Acad Dermatol. 2024;90(3):620-623.
Recent Posts

Living Through TIL Therapy: A Personal Story

You’re Not Out of Options: What to Do When First-Line Melanoma Treatment Fails

Honoring Veterans and Raising Awareness About Skin Cancer

From Service to Survival: A Veteran’s Battle with Melanoma


