Everything Coming up Rosy is not a Health Tune
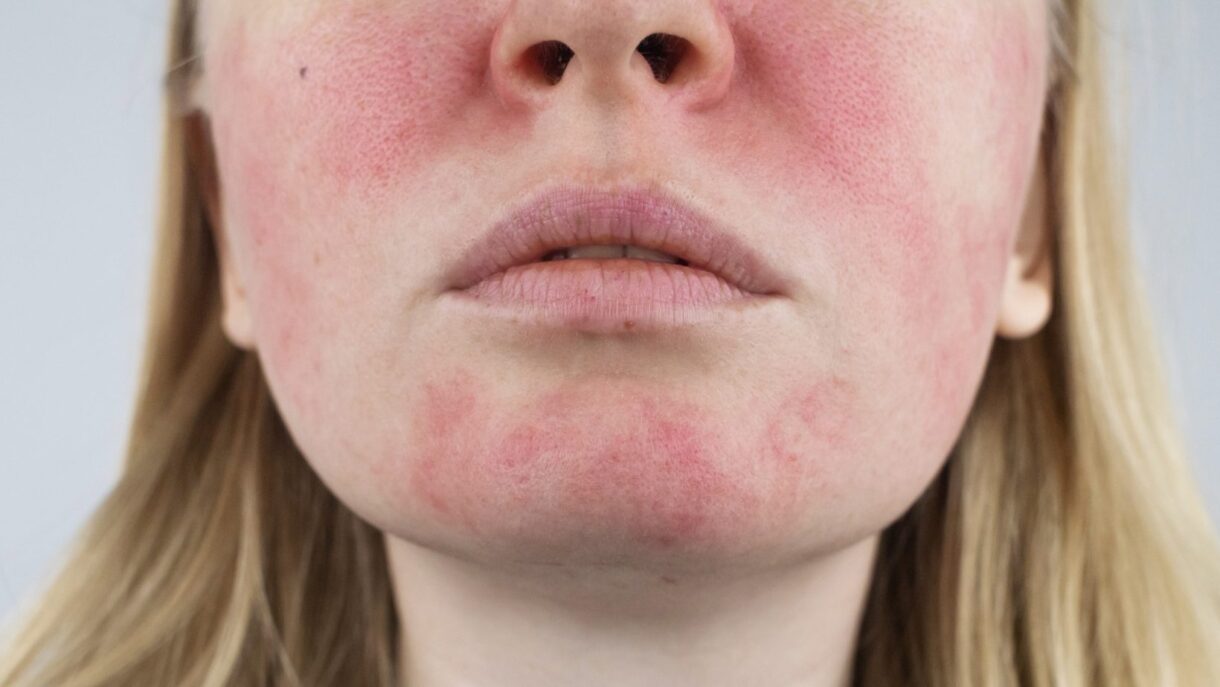
People with the chronic inflammatory skin condition rosacea shouldn’t skip out on check-ups. According to a new research study from…















Copyright © 2014-2022 - AIM at Melanoma Foundation. All rights reserved. Website by RED ZEPHYR DESIGN