Melanoma Int’l Collaboration for Adaptive Trials

What Can We Offer To Patients Who Do Not Respond To Immunotherapy?
Immunotherapy is revolutionary—no doubt. For melanoma, a disease with a single-digit Stage IV survival rate only ten years ago, immunotherapy is a tantalizing breakthrough: A solid percentage of melanoma patients have seen a response.
But for those who don’t see a response—and for those whose response is not durable—the same dismal survival statistics are their reality.
For these thousands of melanoma patients for whom there is no viable treatment, we must change our approach to testing new therapies. Our current clinical trial system tests one or two drugs at a time, which may take multiple years—a slow, inefficient, and ineffective process.
To find the cure for melanoma, we must test therapies in a global, collaborative, adaptive format—a pioneering new model to get effective therapies to patients faster. MICAT is that format. And because the principles of immunotherapy first identified in melanoma have been successfully applied to other cancers, we know that whatever drugs are effective in melanoma will likely be effective in other cancers.
There is a better way. MICAT.
AIM at Melanoma is establishing a global, collaborative, patient-centric, multi-arm clinical trial that will bring effective melanoma therapies to market faster: Melanoma International Collaboration for Adaptive Trials (MICAT).
Traditional Clinic Trials
Controlled Approach, Limited Results
In traditional drug trials, new therapies are tested one or two at a time against the standard of care, a slow approach to finding new treatments.
Patients are randomized to the standard therapy or the new therapy, and regardless of how they are faring, continue with their therapy until the end of the study or, sadly, their death.
Often a study ends with the finding that the new treatment is no better than the standard approach, a disappointing result that costs lives.
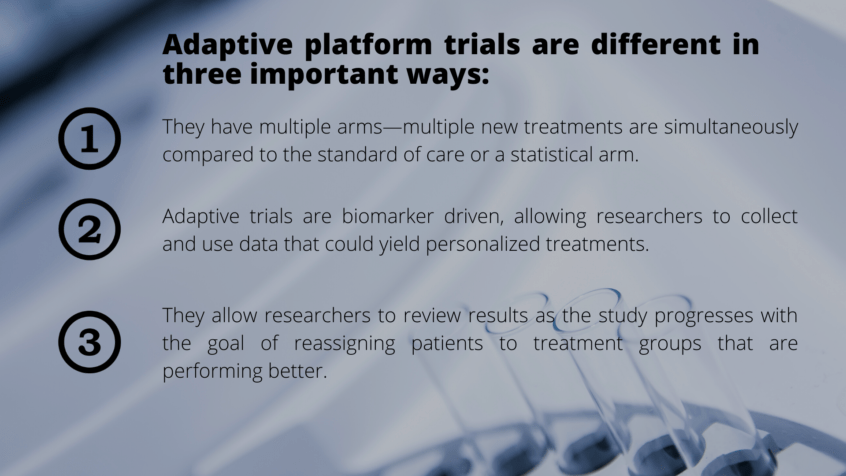
Adaptive platform trials lead to personalized therapies and faster answers on a drug’s efficacy—and may save lives in the process. Melanoma has not had an adaptive platform trial until now. Until MICAT.
Adaptive Platform Trials
A Better, Smarter Way
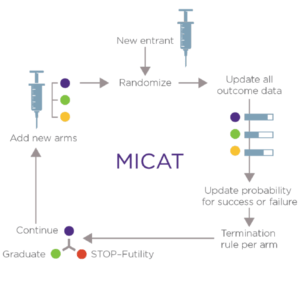
How Will It Work?
- MICAT will use an adaptive design in a modular trial process. Drug candidates will be evaluated in bio-marker defined patient subpopulations to identify the most effective drugs, drug combinations, and treatment sequences for specific melanoma subtypes in the context of standard and emerging biomarkers.
- MICAT will determine the probability that novel therapies will improve progression-free survival. Treatments “graduating” from MICAT will be recommended for advancement, either for Phase III evaluation or potentially for additional evaluation for marketing approval within MICAT. Each recommendation will include biomarker characterization.
- MICAT offers the most efficient, informative evaluation platform to assess all classes of melanoma therapy:
- Interaction of Anti-PD-1 and Anti-CTLA4 with biomarkers
- Checkpoint inhibitors and combinations
- T-cell targeting agents and strategies
MICAT will get the right drug to the right patient at the right time — and will do it faster than traditional clinical trials.
Help Us Find The Cure
Start-up funding for an adaptive platform trial is critical. The estimated cost for MICAT start-up is $5.5M, which includes regulatory submissions for the U.S., the E.U., and Australia and the creation of:
(1) platform design and statistical modeling
(2)drug forecasting system
(3) response adaptive randomization system
Once this initial work is complete, MICAT can welcome industry partners and open the trial. Those who support MICAT will not only help find treatments for melanoma, but also advance the study of cancer biomarkers and aid the search for treatments for multiple cancers.
We need your help. MICAT is seeking financial support for this groundbreaking effort to find the cure for melanoma.

To learn how you can help transform the discovery of effective treatments, please contact:
Alicia Rowell, Vice President
Alicia@AIMatMelanoma.org

