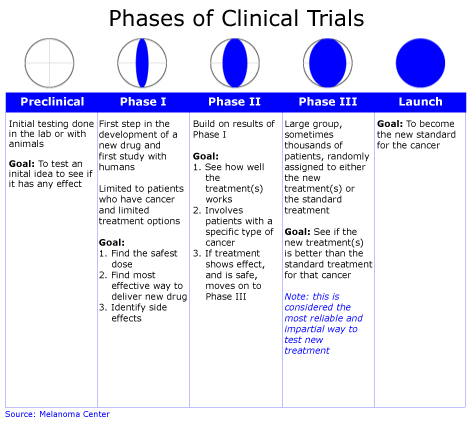
Phase 1: Looking at Safety
Once laboratory studies show that a new approach has promise, a Phase 1 trial can begin. A Phase 1 trial is the first step in testing a new cancer agent in humans. In these studies, researchers look for the best way to give people the new agent (for example, by pill or by injection), how often it should be given, and what the safest dose is. These studies also include special laboratory tests such as blood tests and biopsies to evaluate how the new agent is working in the body. Phase 1 cancer trials involve small groups of people with cancer.
Phase 2: How Well the New Treatment Works
Phase 2 trials continue to test the safety of the new agent and begin to evaluate how well it works against a specific type of cancer. In these trials, the new agent is given to groups of people with a certain type of cancer or related cancers, using the dosage found to be safe in Phase 1 trials. Phase 2 cancer trials usually have less than 100 participants.
Phase 3: Comparing a New Treatment to the Standard Treatment
Phase 3 trials focus on learning how a new treatment compares with standard, or the most widely accepted, treatment. Researchers want to learn whether the new treatment is better than, the same as, or worse than the standard treatment.
In Phase 3 trials, participants have an equal chance to be assigned to one of two or more groups (also called “arms”). The process of assigning participants to groups is called randomization.
In a study with two groups:
group 1 gets the standard treatment (control group)
group 2 gets the new treatment being tested (investigational group)
Phase 4: Continuing Evaluation
Phase 4 trials are used to further evaluate the long-term safety and effectiveness of a treatment. Less common than Phase 1, 2, and 3 trials, Phase 4 trials take place after the new treatment has been approved for standard use.