Sunscreen

What is Sunscreen?
Sunscreen protects the skin from absorbing ultraviolet (UV) rays. Sunscreen products contain one or more UV filters as active ingredients. There are two kinds of UV rays that can affect the skin: UVA and UVB. In the U.S., sunscreen is considered an over-the-counter drug and is regulated by the U.S. Food and Drug Administration.
The preventive benefit of sunscreen was clearly demonstrated in a large Australian randomized trial published in 2010, which showed a 50% decreased melanoma risk by using sunscreen regularly. This study was critical evidence not only for Australia, which has the highest melanoma rate in the world (along with New Zealand), but for all countries. Sunscreen works.
2024 Update: The State of Sunscreen in the U.S.
Our original publication, “Update: The State of Sunscreen in the U.S.,” premiered in 2022. Within this publication, the original text is enriched with the latest updates in pertinent sections, offering readers the latest information.
Two Types of Sunscreen
Sunscreen filters can be broadly classified into two groups: mineral and organic.
Mineral Filters
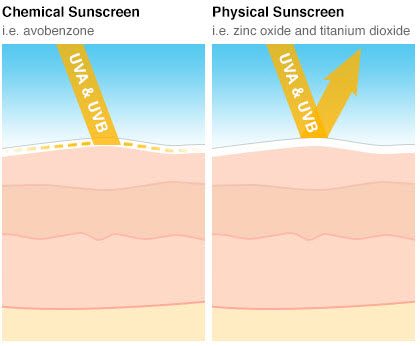
Mineral filters—sometimes called inorganic or physical—work by deflecting or scattering UV light, preventing the UV light from hitting your skin. They are effective at protecting against both UVA and UVB radiation. They are not readily absorbed into your skin. Sunscreens with mineral filters begin to work as soon as they are applied to the skin. The two mineral filters used in U.S. sunscreen are zinc oxide and titanium dioxide.
Organic Filters
Sunscreens with organic—sometimes called chemical—filters work by absorbing UV light. They allow the UV light to hit the skin but transform it into a non-harmful wavelength of light. Unlike physical blockers, organic sunscreens do absorb into the skin. They take about 15 minutes to absorb, so they should be applied 15 minutes prior to any sun exposure in order to work effectively. They often contain multiple organic filters. The filters can be differentiated by the type of rays they absorb: UVA, UVB, or both UVA and UVB. The six organic filters typically used in U.S. sunscreens are avobenzone, homosalate, octinoxate, octisalate, octocrylene, and oxybenzone.
Combination Sunscreens
Some sunscreens are a combination of organic and mineral filters.
Broad Spectrum Sunscreens
Per FDA regulations, sunscreen can only be called “broad spectrum” if it protects against both UVA and UVB. Most U.S. sunscreens combine two or three of the above eight filters to get the best performance. Importantly, only three of these filters—oxybenzone, avobenzone, and zinc oxide—work in the UVA range. While the FDA has approved 16 sunscreen filters for use in the U.S., these eight are most commonly used.
What Does SPF Stand For?
SPF stands for Sun Protection Factor and refers to the level of sunburn protection provided by the sunscreen product. All sunscreens are tested to measure the amount of UV radiation exposure it takes to cause sunburn when using a sunscreen vs. when not using a sunscreen. The higher the SPF, the greater the protection is from UV radiation, and the greater the sunburn protection. Most people assume that SPF relates to a timeframe of protection. For example, it’s a common belief that an SPF of 30 would theoretically allow you to stay in the sun 30 times longer than you could without protection. But the FDA reminds us that the amount of UV exposure isn’t the same as the amount of time spent in the sun. Why? Because radiation exposure is greater at 1 pm than 9 am; greater in Florida than in North Dakota; and greater in summer than in winter. Additionally, your actions affect the amount of UV exposure you receive: You may not apply enough sunscreen, or you may miss areas; you may swim or sweat, which removes sunscreen; your towel or clothing may rub sunscreen off. In all cases, sunscreens are effective for no more than two hours—and all of the above circumstances contribute to how much UV exposure you will receive.
AIM recommends that you use a broad-spectrum sunscreen of SPF 30 or higher daily on all exposed skin and reapply it at least every two hours, as well as after swimming, sweating, or towel drying.
Because SPF values are determined from a test that measures protection against sunburn caused by UVB radiation, SPF values only indicate a sunscreen’s protection from UVB—the kind of radiation that causes sunburn, damages the skin, and can contribute to developing melanoma.
Currently, there is no internationally agreed-upon test for measuring UVA protection in human skin. An estimate is made by a laboratory test in which the proportion of radiation passing through a measured amount of sunscreen is determined.
Here’s how your sun protection looks with each level of SPF:
SPF 15 – filters about 93% of UVB rays
SPF 30 – filters about 97% of UVB rays
SPF 50 – filters about 98% of UVB rays
SPF 100 – filters about 99% of UVB rays
To get the most protection, you want a broad-spectrum sunscreen—these protect against UVB and UVA rays. UVA rays are long enough to reach the skin’s dermal layer, damaging collagen, and elastic tissue. That layer is also where the cells that stimulate skin darkening are found; that’s why UVA rays are considered the dominant tanning rays. (UVA rays are what is found in tanning beds and other tanning devices.) Though many people still think a tan looks healthy, it’s actually a sign of DNA damage—the skin darkens in an imperfect attempt to prevent further injury, which can lead to the cell mutations that can lead to melanoma.
Sunscreen should be just one of the sun protection strategies you use to protect your skin. You also need to cover up with clothing and a hat, seek shade, and try to stay out of the sun during peak hours, which are from 10:00 am to 4:00 pm.
Applying Sunscreen Correctly

Apply enough sunscreen to cover all exposed skin. Most adults need about one ounce — the amount in a shot glass — for full-body protection. Don’t forget to apply it to your ears, hands, feet, the back of your neck and any part of your scalp that isn’t covered by hair. Make sure to apply to the underside of your chin, which can be exposed to reflected sunlight. Remember: Most people don’t apply enough sunscreen, so it’s okay to use more than you think you should. Many people wait to put on sunscreen when already outside, but you should apply it before you go in the sun if you use organic sunscreens, which need approximately 15 minutes to absorb into your skin and begin working. And don’t forget to wear sunscreen even on cloudy days and during the winter months. UV rays can still harm your skin when it’s cloudy outside. Finally, check the expiration date on your sunscreen to make sure it is not out of date. If your sunscreen changes consistency, becomes watery, separates, or changes color, even if it has not expired, it should be discarded. Sunscreen should not be left in direct sunlight or a hot environment like a car, as the heat can break down the chemicals in the sunscreen.
No Sunscreen is Waterproof or Sweatproof

Manufacturers of sunscreen cannot make claims that sunscreens are “waterproof” or “sweatproof.” Instead, sunscreen labels may say “water-resistant” and must specify whether they protect the skin for 40 or 80 minutes of swimming or sweating based on standard testing, according to the FDA. Always reapply sunscreen immediately after swimming, sweating, or toweling.
When To Reapply

Reapply one ounce of sunscreen at least every two hours, even if you haven’t been sweating or swimming. Cover all of your skin that will be exposed to the sun, including often neglected areas like your back, your ears, and behind your knees.
Keep Babies Out of the Sun

Sunscreen may be used on babies older than six months, but the skin on a baby is less mature compared to adults, and infants have a higher surface-area to body-weight ratio compared to older children and adults. Therefore, babies need to be physically protected by keeping them out of the sun and in the shade as much as possible. If there’s no natural shade, create your own with an umbrella, pop-up tent, or the canopy of the stroller. Make sure your baby wears a hat that provides sufficient shade at all times and clothing that covers and protects sensitive skin. Consult your pediatrician before using any sunscreen on your baby.

