Stage IV
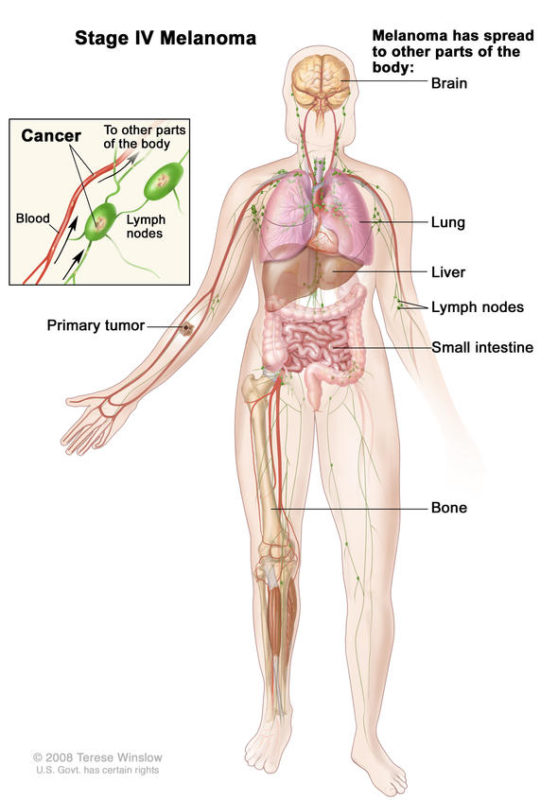
What is Stage IV Melanoma?
Stage IV melanoma has traveled beyond the original tumor site and beyond the regional lymph nodes to more distant areas of the body. The most common sites of metastasis for Stage IV melanoma are distant skin and lymph nodes, then lungs, liver, brain, bone, and/or intestines. The level of serum lactate dehydrogenase (LDH) in the blood may or may not be elevated. The level of LDH is an important sign, as it usually indicates overall tumor burden and possibly how aggressive the tumor is—in other words, it usually indicates the amount of cancer in the body.
- Stage IV melanoma is defined by spread beyond the regional lymph nodes to distant sites
- Important distinctions within Stage IV: Location of different metastases, number and size of tumors, and LDH level
Characteristics of Stage IV Melanoma
Stage IV melanoma is defined by three primary characteristics:
- Location of distant metastases
- Number and size of tumors
- LDH level
Subgroups of Stage IV Melanoma
There are no subgroups of Stage IV melanoma. But in the TNM system (which informs the stage), the M categories and subcategories are helpful to understand the variation in Stage IV disease. (“M” means metastases.)
The M categories and subcategories are based on where the metastases are located and the level of LDH. A higher level of LDH generally correlates with higher tumor burden. Details about the primary tumor, such as thickness and ulceration, no longer matter.
- M1a: the tumor has metastasized to distant skin, soft tissue including muscle, and/or to distant lymph nodes.
- M1a(0): LDH not elevated
- M1a(1): LDH elevated
- M1b: the tumor has metastasized to the lungs (with or without M1a sites of disease)
- M1b(0): LDH not elevated
- M1b(1): LDH elevated
- M1c: the tumor has metastasized to internal organs (with or without M1a or M1b sites of disease)
- M1c(0): LDH not elevated
- M1c(1): LDH elevated
- M1d: the tumor has metastasized to the central nervous system (with or without M1a, M1b, or M1c sites of disease)
- M1d(0): LDH not elevated
- M1d(1): LDH elevated
Stage IV Survival Data
In 2009, one year survival estimates for patients diagnosed with metastatic disease prior to modern immune and targeted therapies were the following:
Distant skin, soft tissue, and/or lymph nodes – 62%
Lung- 53%
Other visceral sites- 33% (1)
Now, we have 5 year data demonstrating 52% survival rate at 5 years with combination Yervoy/Opdivo, 44% with single-agent Opdivo, and 26% with single-agent Yervoy.(2) Median for disease site subgroups were not reached at time of publication. Targeted therapy with Tafinlar/Mekinist inhibition has also demonstrated a 34% survival rate at 5 years.(3)
Why Brain Metastases Are Different
While brain metastases are part of Stage IV, the risk factors, diagnosis, and treatment, are different than other types of metastases and carry a worse prognosis. Learn more about brain metastases.
Why LDH Levels Are Important
Compared with the survival of patients with normal LDH levels, patients with high LDH levels have significantly worse overall survival.
Treatment for Stage IV Melanoma
If you have progressed from an earlier stage diagnosis, your treatment will have included wide local excision and maybe sentinel lymph node biopsy, among other treatments.
For Stage IV specifically, treatments include surgery, systemic therapies, radiation therapy, and clinical trials. The FDA has approved several new drugs that have shown improvements in survival. Many experimental treatments are also under investigation and may be available by enrolling in a clinical trial.
Surgery
Surgery may be performed to remove cancerous tumors or lymph nodes that have metastasized to other areas of the body, if they are few in number and/or are causing symptoms. Surgical options are typically limited for patients with metastatic melanoma and have to be considered carefully in the context of the overall course of the disease.
Systemic Therapies
Drug treatment is recommended for Stage IV melanoma. Only one of the FDA approved agents for metastatic melanoma is delivered locally to specific lesions (T-VEC), but all other treatments are systemic therapies that go through the bloodstream to reach and destroy melanoma cells in the body. Treatments can be divided into immunotherapies, targeted therapies, and chemotherapy. The following are FDA approved treatments for Stage IV melanoma, but some are rarely used.
SINGLE AGENT IMMUNOTHERAPIES
Amtagvi (lifileucel)
Imlygic (talimogene laherparepvec “T-Vec”)
Keytruda (pembrolizumab)
Opdivo (nivolumab)
Proleukin / IL-2 (interleukin-2)
Yervoy (ipilimumab)
SINGLE AGENT TARGETED THERAPIES
Mekinist (trametinib)
Tafinlar (dabrafenib)
Zelboraf (vemurafenib)
COMBINATION THERAPIES
Braktovi (encorafenib) and Mektovi (binimetinib)
Cotellic (cobimetinib) and Zelboraf (vemurafenib)
Opdivo (nivolumab) and Yervoy (ipilimumab)
Opdualag (nivolumab and relatlimab-rmbw)
Tafinlar (dabrafenib) and Mekinist (trametinib)
Tecentriq (atezolizumab) plus Cotellic (cobimetinib) and Zelboraf® (vemurafenib)
CHEMOTHERAPY
DTIC (dacarbazine)
FDA Approved Drugs
To read more about any of the treatments listed above, please go to our
Radiation Therapy
Radiation is used in some situations to slow tumor growth or shrink a tumor in organs where surgery is not possible or is not recommended. It is also used to relieve symptoms caused by tumors, such as in the brain or bone.
Learn more about Radiation Therapy
Clinical Trials
Clinical trials are research studies to evaluate new therapies and improve cancer care. These studies are responsible for most of the advances in cancer prevention, diagnosis, and treatment. These studies offer access to promising novel therapies that are not yet available outside of clinical trials because they are still investigational. You may be eligible to participate in a clinical trial.
Several experimental treatments are currently being tested in clinical trials.
- Immunotherapies: designed to boost the body’s immune response to tumors. Therapies investigated on clinical trial are trying to stimulate the immune system with goals of either more efficacy or less toxicity than currently available treatments. Trials are investigating agents involved in priming and activation of the immune system, infiltration of T cells into tumors, T cell recognition of cancer cells, and improved T cell killing of cancer cells.
- Targeted Therapies: designed to inhibit mutations and pathways that promote the growth and survival of tumor cells. Some clinical trials are designed for patients with specific mutations in their tumors (for example, BRAF), and thus require testing to determine if patients are appropriate for a given therapy.
- Vaccines: developed to target either a common protein found in melanoma or sometimes personalized to proteins specific to a patient’s tumor
- Adoptive Cell Transfer [ACT]: the transfer of immune cells grown and stimulated from a patient’s tumor, often referred to as tumor infiltrating lymphocytes [TIL], although ACT may also use cells that are genetically engineered and not necessarily derived from a patient’s specific TILs.
- Chemotherapy
- Combinations: trials to combine different systemic treatments, as well as trials to test whether combining systemic treatments with surgery, radiation, and other therapies can improve outcomes in patients.
In addition to performing clinical trials to test the safety and effectiveness of new treatments, many investigators are also working to determine why therapies work in some patients but not in others, as well as why they sometimes stop working after initial success. This research depends on the participation of patients in clinical trials, and sometimes in parallel studies that allow researchers to analyze samples of blood, tumor tissue, or other materials. Clinical trials have the potential to help patients who currently have melanoma, but equally important, they have the potential to help melanoma patients in the future. They provide patients with even more options than currently existing therapies, and are generally very well thought through and regulated by the IRB and the FDA to ensure to minimize risk and maximize benefits for patients.
Learn More About Clinical Trials
If you or someone you love has been diagnosed with Stage IV melanoma or is being evaluated for it, this publication is designed to help you and your oncology team evaluate treatment options and identify the different considerations in deciding your treatment course. Using this guide, you and your team can weigh the options to make the decision that is right for you.
What to Ask Your Doctor about Stage IV Melanoma
When your doctor tells you that you have Stage IV melanoma, it can be frightening and overwhelming. But it is important to use the time with all of your doctors to learn as much about your cancer as you can. Your doctors will provide you important information about your diagnosis, prognosis, and treatment options.
It is often helpful to bring a friend or family member with you to your doctor appointments. This person can lend moral support, ask questions, and take notes.
The following questions are those you may want to ask your doctors. Some of the questions are for your medical oncologist, some are for your surgical oncologist, and some for your dermatologist. Remember, it is ALWAYS okay to ask your doctor to repeat or clarify something s/he has said so that you can better understand it. You may find it helpful to print out these questions and bring them with you to your next appointment.
Questions to Ask Your Doctor
To download and print a PDF of Questions To Ask Your Doctor
References:
[1]Balch C M, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. [Internet] 2009 Nov 16 [cited 2020 June 24]; vol. 27,36 (2009): 6199-206. DOI:10.1200/JCO.2009.23.4799[2]Larkin J, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2019 [Internet]. 2019 Oct 17 [cited 2020 June 24]; 381:1535-1546. DOI: 10.1056/NEJMoa1910836
[3]Robert C, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 2019 [Internet]. 2019 Aug 15 [cited 2020 June 24]; 381:626-636. DOI: 10.1056/NEJMoa1904059

